Boston-based Rhythm Pharmaceuticals (NASDAQ:RYTM), a commercial-stage biopharmaceutical company focused on rare genetic diseases of obesity, announced preliminary, unaudited net product revenues for its flagship therapy IMCIVREE (setmelanotide) for the fourth quarter and full year ended December 31, 2025.
Fourth-quarter net product revenues are expected to be approximately $57 million, representing an 11% sequential increase from the third quarter.
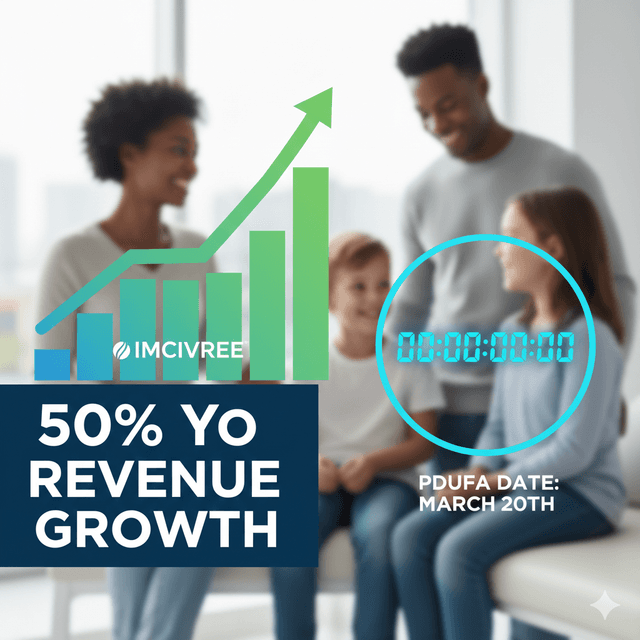
Full-year 2025 net product revenues are anticipated to reach approximately $194 million, reflecting about 50% growth compared with $130 million in 2024.
U.S. sales accounted for approximately 68% of fourth-quarter revenues and 69% of full-year revenues, underscoring continued strong demand in the company's core market for approved indications including POMC, PCSK1, and LEPR deficiency, Bardet-Biedl syndrome, and Alström syndrome.
Looking ahead, Rhythm highlighted several important 2026 milestones.
The supplemental new drug application (sNDA) for IMCIVREE in acquired hypothalamic obesity carries a PDUFA goal date of March 20, 2026.
Topline Phase 3 data are expected in the first quarter of 2026 from a 12-patient Japanese cohort and the EMANATE trial.
Additional catalysts include six-month data from the Prader-Willi syndrome (PWS) program, completion of Part C in the RM-718 study, and potential initiation of a Phase 3 program for the oral MC4R agonist bivamelagon pending regulatory feedback.
The company plans to report final audited financial results for the fourth quarter and full year 2025, along with a detailed business update, in late February 2026.